How Do You Find A Limiting Reagent?
For teaching purposes, let's use the equation:
For the balanced equation C3H6O2S+5O2=>3CO2+3H2O+SO3,
what would the limiting reagent be if 51.9 grams of C3H6O2S were reacted with 42.1 grams of O2?
Step One: Define The Ratio Of The Reagents In Chemical Equation
The ratio of the reagents is given by the original balanced equation:
C3H6O2S+5O2=>3CO2+3H2O+SO3
There is 1 unit of C3H6O2S while there are 5 units of O2
Therefore, the ratio is 1:5
Step Two: You Must Find The Molar Mass Of Each Reagent
A Mole is equal to 6e23 molecules and is the universal unit used for measuring amounts of chemical substances
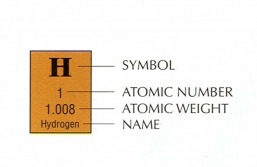
The Atomic Mass Of An Element is the same as the Molar Mass (or Atomic Weight) and is written on the Periodic Table of Elements. (See Labeled Image At Left)
The Molar Mass Of a Compound is found in three steps. To represent the steps, let's use the chemical formula C3H6O2S
Step One: Identify the amount of each element that is present in the formula.
C * 3: There are 3 carbon written in the formula
H * 6: There are 6 hydrogen written in the formula
O * 2: There are 2 oxygen written in the formula
S * 1: There is 1 sulfur written in the formula
Step Two: Look up the Atomic Masses of each element and multiply them by the amount of each element present
12 * 3 = 36 :The Atomic Mass of carbon is 12 and there are 3 carbons written in the formula
1 * 6 = 6 :The Atomic Mass of hydrogen is 1 and there are 6 hydrogen written in the formula
16 * 2 = 32 :The Atomic Mass of oxygen is 16 and there are 2 oxygen written in the formula
32 * 1 = 32 :The Atomic Mass of sulfur is 32 and there is 1 sulfur written in the formula
Step Three: Add the products of Step Two together to discover the Molar Mass of the entire compound
36 + 6 + 32 + 32 = 106 Grams / Mole
Following these same steps, you will discover the Atomic Mass of O2, the second part of the equation, to be 32
Step Three: You Must Convert Grams To Moles
Returning to the problem as posted above, you are trying to find the limiting reagent if 51.9 grams of C3H6O2S were reacted with 42.1 grams of O2
Because you are given the amount in grams you must convert grams to moles.
Converting grams to moles in these types of equations is quite simple;
You must simply Divide the amount of grams by the Molar Mass of the Compound (as calculated in step two):
51.9 grams of C3H6O2S: 42.1 grams of O2:
51.9 grams = 0.48moles 42.1 grams = 1.31moles
106 grams/mole 32 grams/mole
Step Four: Use Your Step Three Results To Identify The Limiting Reagent
Use these amounts you discovered to determine the amount of product that can be made based on the ratio of moles of product produced to moles of a specific reagent used.
C3H6O2S+5O2=>3CO2+3H2O+SO3
Considering the balanced equation, let's use production of CO2 as an example.
If you use 1 mole of C3H6O2S+5O2 you get 3 moles of CO2
If you us 5 moles of O2, you get 3 moles of CO2
Noting your results from step three, you have 0.48 moles of C3H6O2S+5O2 and 1.31 moles of O2
You can use these amount to determine the amount of product that can be made
0.48 * (3 moles of CO2 produced / 1 mole of C3H6O2S used) = 1.44
1.31 * (3 moles of CO2 produced / 5 moles of O2 used) = 0.786
Because the amount of oxygen gives you a lesser amount of product, the limiting reagent is O2
